In this guide we explain how an automotive 12 volt car batteries work, from the chemistry to the internal mechanisms to help you understand how your car gets it's initial electrical power. A battery will typically maintain a voltage around 12.6 to 12.8 volts when not under load. Important: Always wear protective gloves and eyewear when working around a car battery. Near touch your eyes, skin or clothing when working with a battery because it will have acid residue around the outside case and near the terminals.
Introduction
Your car battery is designed to store electrical power which is used to start the engine when the key is turned on to initiate the operation of the vehicle, also to operate the accessories in the electrical system such as the light, radio or wipers when the engine is not running.
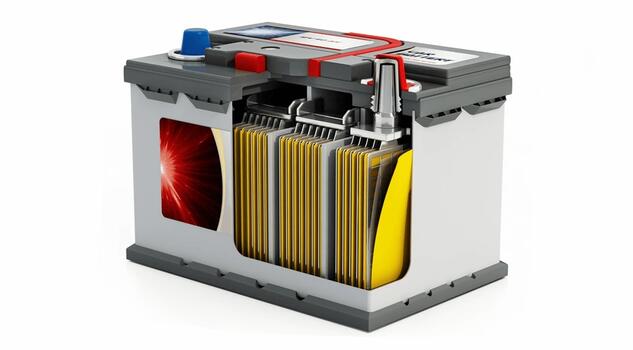
Lead-Acid Battery
- Case: Made from polypropylene plastic, this case contains the internal components and provides protection.
- Plates: Composed of lead and lead oxide, these create electricity within the battery.
- Electrolyte: A mixture of sulfuric acid and water, this solution facilitates the flow of electrons by conducting ions.
- Separators: Insulating material between plates, preventing internal short circuits.
- Terminals: Connects the battery plates and to the vehicle’s positive and negative battery cables.
A car battery is a rechargeable unit in the SLI (starting-lighting-ignition) variety which is lead acid based and is the oldest style of rechargeable electrical supplies. 12 Volt batteries are divided into 6 separate cells, each cell producing about 2.1 volts and when combined create up to 12.6 volts. When a battery powers a device, a chemical reaction occurs between the sulfuric acid in the electrolyte and the lead plates. This reaction forms lead sulfate and releases electrons, generating an electric current. As the state of charge decreases the voltage and amperage it produces also decreases.
A battery is equipped with a positive and negative terminal from which two cables are attached. Voltage is released through the positive terminal (red) and returned to the negative terminal (black). These cables route positive power (red +) from the terminal to the power distribution center or fuse panel and negative (black - ) ground to the engine block and the car body/frame.
Alternator Charging
Combustion engine cars have an alternator mounted near the front of the engine, which charges the battery while the engine is running by reversing the batteries chemical reaction, converting electrical energy back into chemical energy. The battery is discharged after the engine starter is used by converting chemical energy into electrical energy.
What Goes Wrong?
A battery subjected to a complete discharge will fail prematurely unless it is a deep cycle battery. When a battery starts to go bad one or more of the cells with short out which will decrease the battery output voltage by 2.1 volts, which coined the term (dead cell). Also, a bad battery can give off hydrogen gasses which you can smell, which can explode the battery if ignited. This can happen with any kind of spark above or near the battery.
Also, avoid sparks when jumping a dead battery, always connect a ground cable last away from the battery like to an engine bracket etc, here is how to jump a battery correctly. When replacing a battery it is best to neutralize the area with backing soda and rinse with a garden hose and allowed to dry.
Never allow to a battery to "be loose" and not to be mounted securely (battery hold down), because it can be jostled around while breaking internal plates and connections causing a battery failure. Car's have been know to "cut out" and lose all electrical power while driving.
Battery Care
- A battery must have a rest period or "downtime" to allow the plates inside the cells time to cool.
- Store a battery away from the cement floor as it can drain the battery.
- Always store a battery fully charged, batteries do not like to sit with a low charge and will cause premature failure. A deep cycle battery can better withstand this kind of use.
- Disconnect the battery if the car is going to sit for over 3 months.
- Car batteries are hazardous material, and must be disposed of at a local parts store or recycle center.
Battery Performance
There are three main factors that will affect the batteries performance which will shorten the life of the battery which is typically between 3 to 5 years depending on the battery build quality.
1. Temperature: Extreme hot or cold can shorten the life of the battery
2. Age: Time causes the integrity of the chemicals and materials inside the battery to degrade.
3. Downtime: A battery must have time to "rest" to help cool the plate materials.
4. Bad Connections: Loose or corroded battery terminals can cause the battery to "overwork" which leads to excessive heat.
5. If the battery warning light has been on, it means the battery is not charging and will go dead, you should test the alternator output and replace the alternator if it's not charging.
Credits
This guide knowledge base was created by the 2CarPros Team, and by Ken Lavacot: Automobile repair shop owner and certified master automobile technician of over 30 years. If you have question or need help please ask one of our experts we are happy to help. Please visit our 2CarPros YouTube Channel.